

*** INCOMPLETE ***
This chapter will cover the physics behind the operation of semiconductor devices and show how these principles are applied in several different types of semiconductor devices. Subsequent chapters will deal primarily with the practical aspects of these devices in circuits and omit theory as much as possible.
"Here be dragons."
Physicist Enrico Fermi, drawing attention to the nucleus of an atom after a lecture on atomic structure
To say that the invention of semiconductor devices was a revolution would not be an exaggeration. Not only was this an impressive technological accomplishment, but it paved the way for developments that would indelibly alter modern society. Semiconductor devices made possible miniaturized electronics, including computers, certain types of medical diagnostic and treatment equipment, and popular telecommunication devices, to name a few applications of this technology.
But behind this revolution in technology stands an even greater revolution in general science: the field of quantum physics. Without this leap in understanding the natural world, the development of semiconductor devices (and more advanced electronic devices still under development) would never have been possible. Our investigation into how and why electronic devices work properly begins with this important area of study.
Many of us have seen diagrams of atoms that look something like this:
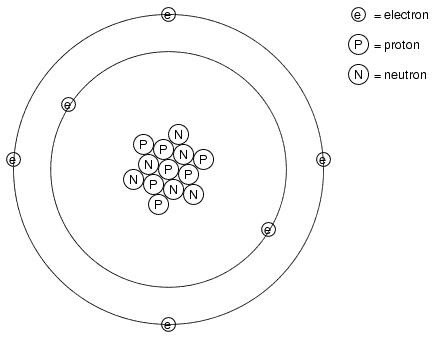
Tiny particles of matter called protons and neutrons make up the center of the atom, while electrons orbit around not unlike planets around a star. The nucleus carries a positive electrical charge, owing to the presence of protons (the neutrons have no electrical charge whatsoever), while the atom's balancing negative charge resides in the orbiting electrons. The negative electrons tend to be attracted to the positive protons just as planets are gravitationally attracted toward whatever object(s) they orbit, yet the orbits are stable due to the electrons' motion. We owe this popular model of the atom to the work of Ernest Rutherford, who around the year 1911 experimentally determined that atoms' positive charges were concentrated in a tiny, dense core rather than being spread evenly about the diameter as was proposed by an earlier researcher, J.J. Thompson.
While Rutherford's atomic model accounted for experimental data better than Thompson's, it still wasn't perfect. Further attempts at defining atomic structure were undertaken, and these efforts helped pave the way for the bizarre discoveries of quantum physics. Today our understanding of the atom is quite a bit more complex. However, despite the revolution of quantum physics and the impact it had on our understanding of atomic structure, Rutherford's solar-system picture of the atom embedded itself in the popular conscience to the point that it persists in some areas of study even when inappropriate.
Read this short description of electrons in an atom, taken from a popular electronics textbook:
Orbiting negative electrons are therefore attracted toward the positive nucleus, which leads us to the question of why the electrons do not fly into the atom's nucleus. The answer is that the orbiting electrons remain in their stable orbit due to two equal but opposite forces. The centrifugal outward force exerted on the electrons due to the orbit counteracts the attractive inward force (centripetal) trying to pull the electrons toward the nucleus due to the unlike charges.
In keeping with the Rutherford model, this author casts the electrons as solid chunks of matter engaged in circular orbits, their inward attraction to the oppositely charged nucleus balanced by their motion. The reference to "centrifugal force" is technically incorrect (even for orbiting planets), but is easily forgiven due to its popular acceptance: in reality, there is no such thing as a force pushing any orbiting body away from its center of orbit. It only seems that way because a body's inertia tends to keep it traveling in a straight line, and since an orbit is a constant deviation (acceleration) from straight-line travel, there is constant inertial opposition to whatever force is attracting the body toward the orbit center (centripetal), be it gravity, electrostatic attraction, or even the tension of a mechanical link.
The real problem with this explanation, however, is the idea of electrons taking circular orbits in the first place. It is a verifiable fact that accelerating electric charges emit electromagnetic radiation, and this fact was known even in Rutherford's time. Since orbiting motion is a form of acceleration (the orbiting object in constant acceleration away from normal, straight-line motion), electrons in an orbiting state should be throwing off radiation like mud from a spinning tire. Electrons accelerated around circular paths in particle accelerators called synchrotrons are known to do this, and the result is called synchrotron radiation. If electrons are losing energy in this way, their orbits should eventually decay, resulting in a collision with the positively charged nucleus. However, this doesn't ordinarily happen within atoms. Indeed, electron "orbits" are remarkably stable over a wide range of conditions.
Furthermore, experiments with "excited" atoms demonstrated that electromagnetic energy emitted by an atom occurs only at certain, definite frequencies. Atoms that are "excited" by outside influences such as light are known to absorb that energy and return it as electromagnetic waves of very specific frequencies. According to Rutherford's solar-system atomic model and the laws of classical physics, atoms should be able to return energy over a virtually limitless range of frequencies rather than a select few.
A pioneering researcher by the name of Neils Bohr attempted to improve upon Rutherford's model after studying in Rutherford's laboratory for several months in 1912. Trying to harmonize the findings of other physicists (most notably, Max Planck and Albert Einstein), Bohr suggested that each electron possessed a certain, specific amount of energy, and that their orbits were likewise quantized such that they could only occupy certain places around the nucleus, somewhat like marbles fixed in circular tracks around the nucleus rather than the free-wheeling planetoids they were formerly visualized as. In deference to the laws of electromagnetics and accelerating charges, Bohr referred to these "orbits" as stationary states so as to escape the implication that they were in motion.
While Bohr's ambitious attempt at reframing the structure of the atom in terms that agreed closer to experimental results was a milestone in physics, it was by no means complete. His mathematical analyses produced better predictions of experimental events than analyses belonging to previous models, but there were still some unanswered questions as to why electrons would behave in such strange ways. The assertion that electrons existed in stationary, quantized states around the nucleus certainly accounted for experimental data better than Rutherford's model, but he had no idea what would force electrons to manifest those particular states. The answer to that question had to come from another physicist, Louis de Broglie, about a decade later.
De Broglie proposed that electrons, like photons (particles of light) manifested both particle-like and wave-like properties. Building on this proposal, he suggested that an analysis of orbiting electrons from a wave perspective rather than a particle perspective might make more sense of their quantized nature. Indeed, this was the case, and another breakthrough in understanding was reached.
The atom according to de Broglie consisted of electrons existing in the form of standing waves, a phenomenon well known to physicists in a variety of forms. Like the plucked string of a musical instrument vibrating at a resonant frequency, with "nodes" and "antinodes" forming along its length, de Broglie envisioned electrons around atoms standing as waves bent around a circle:
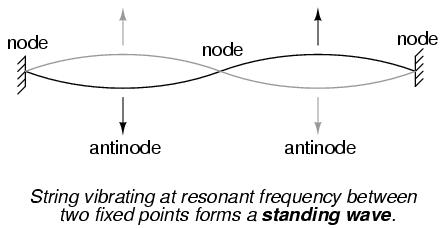
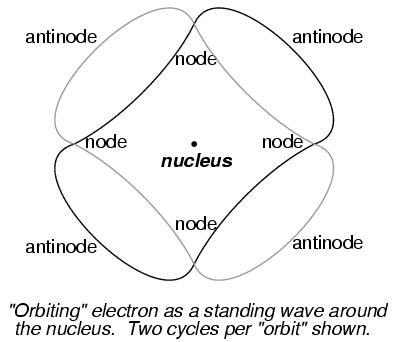
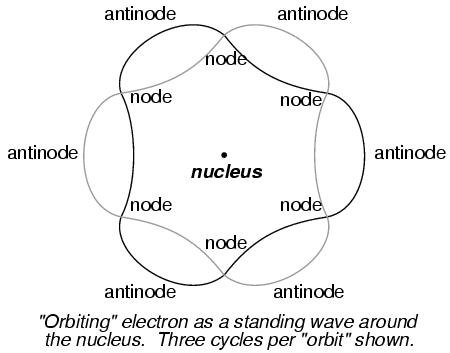
DeBroglie's hypothesis gave both mathematical support and a convenient physical analogy to account for the quantized states of electrons within an atom.


